USP <825> Radiopharmaceutical Modular Cleanrooms
Many of today’s most advanced radiopharmaceuticals use short-lived isotopes with half-lives of just hours, resulting in transportation challenges associated with traditional centralized manufacturing.
Modular Devices is redefining radiopharm manufacturing with modular, GMP-compliant USP <825> cleanrooms that enable manufacturers, hospitals, and cancer centers to produce isotopes at point of care.
This shift to local, on-demand production empowers faster, more accurate diagnoses and timeline, patient-specific treatment—reducing logistical barterers, minimizing waste, and transforming how radiopharmaceuticals are delivered to the patient who need them the most.
Modular Cleanrooms for
On-Site Radiopharmaceutical Production
Our state-of-the-art turnkey modular cleanrooms arrive fully validated and ready for immediate use. Designed to meet USP <825> standards, they enable radiopharmaceutical companies to accelerate clinical trials, scale production, and reduce supply chain risks.
Key Benefits of Pre-built USP <825> Radiopharm Cleanrooms
- Eliminates losses from isotope decay – On-site production ensures isotopes with short half-lives are delivered in a timely manner, maximizing diagnostic and therapeutic effectiveness.
- Enables true point-of-care production – Manufacture radiopharmaceuticals directly where patients are treated, cutting transport delays and logistical complexity.
- Supports personalized, patient-specific therapies – Flexible, small-batch production allows customization by isotope type, dose, and timing for each patient.
- Accelerates diagnosis and treatment – Local, on-demand production streamlines workflows and enables same-day imaging or therapy.
- Compliant, turnkey cleanroom solutions – Fully integrated, modular USP <825> environments designed for rapid deployment and regulatory confidence.
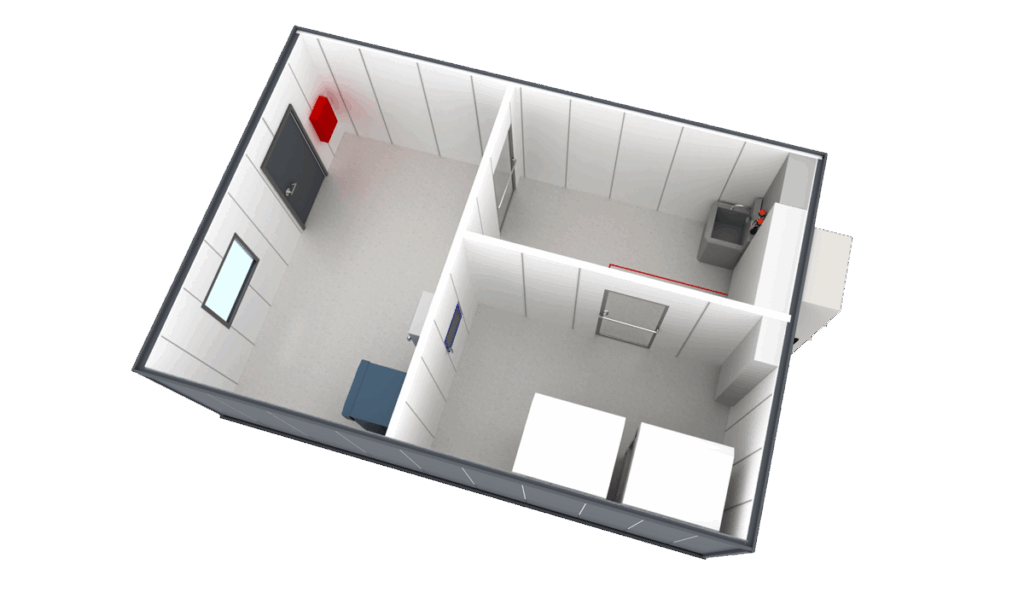

Built To Meet Radiopharm Cleanroom Classifications
With more than 35 years of experience in cleanroom design, manufacturing and installation, we deliver solutions that adapt to each client’s unique needs. From rigidwall and hardwall cleanrooms to custom hybrid designs, our team ensures regulatory compliance and project efficiency.
Advantages of Panel-Built Cleanrooms
- Unmatched compliance – ISO, cGMP, and USP <825> requirements
- Configurable and scalable – Can be adapted, disassembled, and relocated as necessary
- Faster timelines – Parallel-path site prep during fabrication for reduced project times of 50%-plus
- Flexible integration – Can be built as a stand-alone facility or integrated into existing infrastructure
- In-house expertise – Design, engineering, constriction, project management, and compliance teams all under one roof
Why Choose Modular Devices?
Your Partner for Radiopharmaceutical Cleanroom Solutions
35-plus years delivering compliant spaces for health care and life sciences
Deep expertise in USP <825>, cGMP, and ISO cleanroom standards
In-house engineering, construction and fabrication at our 100,000-square-foot factory in Indianapolis, Indiana
Flexible deployment, including leased, purchased, and fully custom builds
Trusted by leading innovators worldwide
Learn about industry trends
Ready for a Modular Radiopharm Cleanroom?
Your unique challenges can be solved by a conversation with one of our industry experts.