Radiopharmaceuticals play a critical role in modern diagnostics and targeted therapies, yet they introduce a level of complexity that extends far beyond traditional sterile compounding. Their radioactive nature, extremely short half-lives, and time-sensitive preparation requirements demand a regulatory framework that prioritizes safety for both the patient and clinician, as well as operational precision. USP <825> was created to address these unique challenges and provide consistent standards for radiopharmaceutical preparation, compounding, dispensing, and repackaging across healthcare settings.
Radiopharmaceuticals play a critical role in modern diagnostics and targeted therapies, yet they introduce a level of complexity that extends far beyond traditional sterile compounding. Their radioactive nature, extremely short half-lives, and time-sensitive preparation requirements demand a regulatory framework that prioritizes safety for both the patient and clinician, as well as operational precision. USP <825> was created to address these unique challenges and provide consistent standards for radiopharmaceutical preparation, compounding, dispensing, and repackaging across healthcare settings.
As the industry moves toward decentralized, point-of-care production models, USP <825> has become increasingly relevant, especially for facilities that must prepare radiopharmaceuticals as close as possible to the time and location of administration.
Understanding the Purpose of USP <825>
USP <825> exists to ensure that radiopharmaceuticals are prepared under conditions that preserve sterility, accuracy, and quality while minimizing radioneon exposure to staff. Unlike conventional sterile drugs, many radiopharmaceuticals decay rapidly, making delays in preparation or transport unacceptable. A product that arrives late or loses potency due to radioactive decay may be clinically ineffective, regardless of the efficacy of production.
By establishing clear expectations for environmental controls, aseptic technique, documentation, and quality oversight, USP <825> helps healthcare facilities reduce variability in practice and ensure that every dose administered meets both safety and efficacy standards.
Scope and Applicability in a Changing Care Model
USP <825> applies to any healthcare facility or pharmacy involved in the handling of radiopharmaceuticals, including hospital pharmacies, nuclear medicine departments, PET imaging centers, and outsourcing facilities. Importantly, the chapter does not distinguish between centralized or decentralized operations. Whether the agents are prepared at a centralized manufacturing facility or in a local production space, the same core principles of control, cleanliness, and documentation apply.
This flexibility is particularly important as healthcare systems adapt to the realities of short half-life radiopharmaceuticals. For many PET Tracers and therapeutic agents, centralized manufacturing is simply not practical. As a result, facilities are increasingly bringing production close to point-of-care, which introduces new challenges related to space constraints, infrastructure limitations, and regulatory compliance.
Environmental Control as a Core of Compliance
At the heart of USP <825> is the expectation that radiopharmaceuticals are prepared in controlled, classified environments that support aseptic processing. These environments must limit particulate and microbial contamination while accommodating shielding, specialized equipment, and radiation safety protocols.
Primary engineering controls such as laminar airflow hoods, isolators, or shielded hot cells are essential, but they cannot function in isolation. USP <825> requires that these systems operate within appropriately designed secondary environments—spaces that maintain consistent airflow, filtration, pressure relationships, and cleanliness. Without this foundation, even the most advanced equipment cannot reliably deliver compliant outcomes.
The impact of Short Half-Lives on Facility Design
Short radioactive half-lives fundamentally reshape how radiopharmaceuticals are manufactured and delivered. When products decay within hours—or even minutes—every step in the process becomes time-critical. Facilities must be able to prepare doses quickly, safely, and consistently, often within close proximity to imaging or treatment areas.
This reality has driven the shift towards decentralized radiopharmaceutical manufacturing, where production occurs on-site or near-site rather than in a distant centralized facility. While this model improves efficiency and preserves product potency, it also requires infrastructure that can be deployed in nontraditional spaces without compromising compliance.
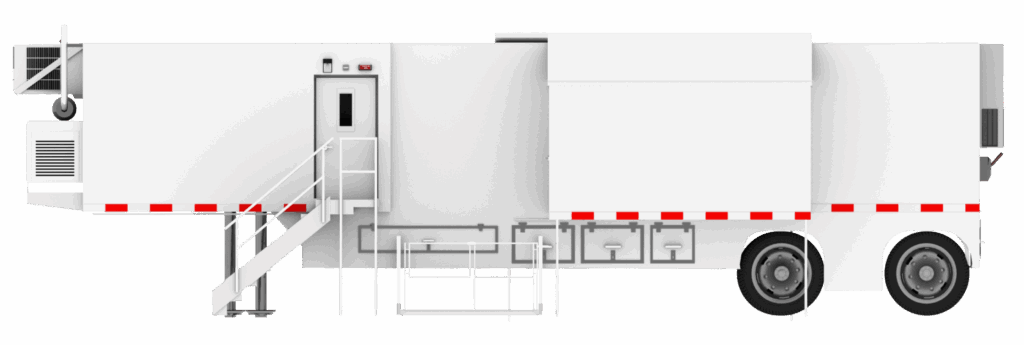
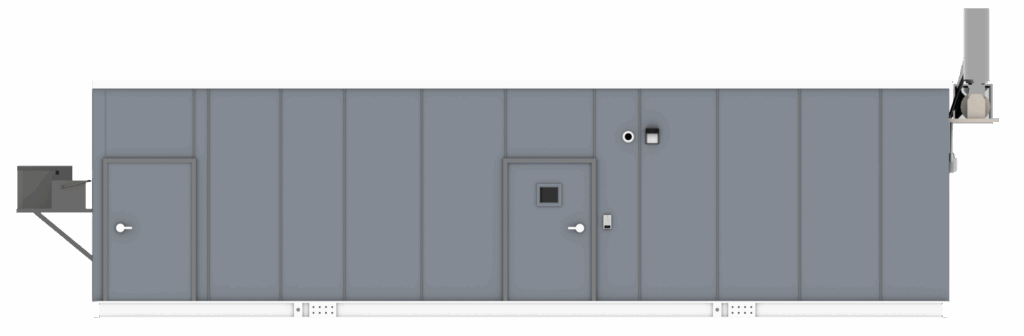
 Mobile and Modular Cleanrooms: Enabling Decentralized Compliance
Mobile and Modular Cleanrooms: Enabling Decentralized Compliance
Mobile cleanrooms have emerged as a powerful solution for healthcare facilities navigating these constraints. Designed to deliver ISO-classified, validated environments in a compact and deployable format, mobile and modular cleanrooms allow radiopharmaceutical preparation to occur precisely where it is needed without extensive construction or disruption to existing operations.
For facilities managing short half-life products, mobile cleanrooms support rapid preparation and administration while maintaining the environmental controls required under USP <825>. They integrate seamlessly with primary engineering controls and can be configured to accommodate shielding, workflow separation, and radiation safety considerations. Just as importantly, mobile and modular radiopharm cleanrooms provide documented inspection-ready environments that align with regulatory expectations.
Mobile and commercial modular cleanrooms not only shorten timelines from production to delivery for nuclear agents; they shorten overall program deadlines. As opposed to traditional cleanroom construction, which can take more than a year and affect on-site productivity, mobile and commercial cleanrooms are constructed off-site and delivered ready for use in just weeks to months.
Cleanrooms as the Foundation of USP <825> Readiness>
USP <825> is often viewed through the lens of procedures and documentation, but in practice, compliance begins with the physical environment. Cleanrooms, whether permanent, commercial modular, or mobile—establish the conditions necessary for aseptic technique, reliable beyond-use dating, and consistent quality outcomes.
A well-designed cleanroom enables staff to work efficiently and safely, supports, standardized workflows, and reduces risk of contamination or compliance gaps. As regulatory scrutiny increases and radiopharma therapies continue to expand, facilities that invest in cleanroom infrastructure are better positioned to adapt, scale, and succeed.
Preparing the Future of Radiopharmaceutical Care
The growth of nuclear medicine, PET imaging, and targeted radiopharmaceutical therapies is accelerating, and USP <825> will remain a central framework guiding safe and compliant practice. Facilities that embrace flexible cleanroom solutions can meet today’s requirements while preparing for tomorrow’s innovations.
By aligning cleanroom design with USP <825> standards and the realities of decentralized manufacturing, pharmacies and healthcare facilities can ensure that radiopharmaceuticals are prepared safely, delivered efficiently, and administered at their full therapeutic potential.
Modular Devices is proud to offer commercial modular cleanrooms designed specifically to meet USP <825> regulations for radiopharm manufacturing and distribution. Learn more.